標準電極電勢表,是指半反應按電極電勢由低到高排序,可十分簡明地判斷氧化還原反應的方向。標準電極電勢是可逆電極在標準狀態及平衡態時的電勢,也就是標準態時的電極電勢。標準電極電勢有很大的實用價值,可用來判斷氧化劑與還原劑的相對強弱,判斷氧化還原反應的進行方向,計算原電池的電動勢、反應自由能、平衡常數,計算其他半反應的標準電極電勢,等等。
基本介紹
- 中文名:標準電極電勢表
- 外文名:Tables of Standard Electrode Potentials
- 套用學科:電化學等
- 適用領域範圍:化學滴定分析,電池等
電極電勢產生
公式
電極電勢表
在酸性溶液中(298K)
電對 | 方程式 | Eq/V |
Li(I)-(0) | Li++e-=Li | -3.0401 |
K(I)-(0) | K++e-=K | -2.931 |
Rb(I)-(0) | Rb++e-=Rb | -2.927 |
Cs(I)-(0) | Cs++e-=Cs | -2.925 |
Ba(II)-(0) | Ba2++2e-=Ba | -2.912 |
Sr(II)-(0) | Sr2++2e-=Sr | -2.89 |
Ca(II)-(0) | Ca2++2e-=Ca | -2.868 |
Na(I)-(0) | Na++e-=Na | -2.71 |
La(III)-(0) | La3++3e-=La | -2.379 |
Mg(II)-(0) | Mg2++2e-=Mg | -2.372 |
Ce(III)-(0) | Ce3++3e-=Ce | -2.336 |
H(0)-(-I) | H2(g)+2e-=2H- | -2.23 |
Al(III)-(0) | AlF63-+3e-=Al+6F- | -2.069 |
Th(IV)-(0) | Th4++4e-=Th | -1.899 |
Be(II)-(0) | Be2++2e-=Be | -1.847 |
U(III)-(0) | U3++3e-=U | -1.798 |
Hf(IV)-(0) | HfO2++2H++4e-=Hf+H2O | -1.724 |
Al(III)-(0) | Al3++3e-=Al | -1.662 |
Ti(II)-(0) | Ti2++2e-=Ti | -1.630 |
Zr(IV)-(0) | ZrO2+4H++4e-=Zr+2H2O | -1.553 |
Si(IV)-(0) | [SiF6]2-+4e-=Si+6F- | -1.24 |
Mn(II)-(0) | Mn2++2e-=Mn | -1.185 |
Cr(II)-(0) | Cr2++2e-=Cr | -0.913 |
Ti(III)-(II) | Ti3++e-=Ti2+ | -0.9 |
B(III)-(0) | H3BO3+3H++3e-=B+3H2O | -0.8698 |
*Ti(IV)-(0) | TiO2+4H++4e-=Ti+2H2O | -0.86 |
Te(0)-(-II) | Te+2H++2e-=H2Te | -0.793 |
Zn(II)-(0) | Zn2++2e-=Zn | -0.7618 |
Ta(V)-(0) | Ta2O5+10H++10e-=2Ta+5H2O | -0.750 |
Cr(III)-(0) | Cr3++3e-=Cr | -0.744 |
Nb(V)-(0) | Nb2O5+10H++10e-=2Nb+5H2O | -0.644 |
As(0)-(-III) | As+3H++3e-=AsH3 | -0.608 |
U(IV)-(III) | U4++e-=U3+ | -0.607 |
Ga(III)-(0) | Ga3++3e-=Ga | -0.549 |
P(I)-(0) | H3PO2+H++e-=P+2H2O | -0.508 |
P(III)-(I) | H3PO3+2H++2e-=H3PO2+H2O | -0.499 |
*C(IV)-(III) | 2CO2+2H++2e-=H2C2O4 | -0.49 |
Fe(II)-(0) | Fe2++2e-=Fe | -0.447 |
Cr(III)-(II) | Cr3++e-=Cr2+ | -0.407 |
Cd(II)-(0) | Cd2++2e-=Cd | -0.4030 |
Se(0)-(-II) | Se+2H++2e-=H2Se(aq) | -0.399 |
Pb(II)-(0) | PbI2+2e-=Pb+2I- | -0.365 |
Eu(III)-(II) | Eu3++e-=Eu2+ | -0.36 |
Pb(II)-(0) | PbSO4+2e-=Pb+SO42- | -0.3588 |
In(III)-(0) | In3++3e-=In | -0.3382 |
Tl(I)-(0) | Tl++e-=Tl | -0.336 |
Co(II)-(0) | Co2++2e-=Co | -0.28 |
P(V)-(III) | H3PO4+2H++2e-=H3PO3+H2O | -0.276 |
Pb(II)-(0) | PbCl2+2e-=Pb+2Cl- | -0.2675 |
Ni(II)-(0) | Ni2++2e-=Ni | -0.257 |
V(III)-(II) | V3++e-=V2+ | -0.255 |
Ge(IV)-(0) | H2GeO3+4H++4e-=Ge+3H2O | -0.182 |
Ag(I)-(0) | AgI+e-=Ag+I- | -0.15224 |
Sn(II)-(0) | Sn2++2e-=Sn | -0.1375 |
Pb(II)-(0) | Pb2++2e-=Pb | -0.1262 |
*C(IV)-(II) | CO2(g)+2H++2e-=CO+H2O | -0.12 |
P(0)-(-III) | P(白磷)+3H++3e-=PH3(g) | -0.063 |
Hg(I)-(0) | Hg2I2+2e-=2Hg+2I- | -0.0405 |
Fe(III)-(0) | Fe3++3e-=Fe | -0.037 |
H(I)-(0) | 2H++2e-=H2 | 0.0000 |
Ag(I)-(0) | AgBr+e-=Ag+Br- | 0.07133 |
S(II.V)-(II) | S4O62-+2e-=2S2O32- | 0.08 |
*Ti(IV)-(III) | TiO2++2H++e-=Ti3++H2O | 0.1 |
S(0)-(-II) | S+2H++2e-=H2S(aq) | 0.142 |
Sn(IV)-(II) | Sn4++2e-=Sn2+ | 0.151 |
Sb(III)-(0) | Sb2O3+6H++6e-=2Sb+3H2O | 0.152 |
Cu(II)-(I) | Cu2++e-=Cu+ | 0.153 |
Bi(III)-(0) | BiOCl+2H++3e-=Bi+Cl-+H2O | 0.1583 |
S(VI)-(IV) | SO42-+4H++2e-=H2SO3+H2O | 0.172 |
Sb(III)-(0) | SbO++2H++3e-=Sb+H2O | 0.212 |
Ag(I)-(0) | AgCl+e-=Ag+Cl- | 0.22233 |
As(III)-(0) | HAsO2+3H++3e-=As+2H2O | 0.248 |
Hg(I)-(0) | Hg2Cl2+2e-=2Hg+2Cl-(飽和KCl) | 0.26808 |
Bi(III)-(0) | BiO++2H++3e-=Bi+H2O | 0.320 |
U(VI)-(IV) | UO22++4H++2e-=U4++2H2O | 0.327 |
C(IV)-(III) | 2HCNO+2H++2e-=(CN)2+2H2O | 0.330 |
V(IV)-(III) | VO2++2H++e-=V3++H2O | 0.337 |
Cu(II)-(0) | Cu2++2e-=Cu | 0.3419 |
Re(VII)-(0) | ReO4-+8H++7e-=Re+4H2O | 0.368 |
Ag(I)-(0) | Ag2CrO4+2e-=2Ag+CrO42- | 0.4470 |
S(IV)-(0) | H2SO3+4H++4e-=S+3H2O | 0.449 |
Cu(I)-(0) | Cu++e-=Cu | 0.521 |
I(0)-(-I) | I2+2e-=2I- | 0.5355 |
I(0)-(-I) | I3-+2e-=3I- | 0.536 |
As(V)-(III) | H3AsO4+2H++2e-=HAsO2+2H2O | 0.560 |
Sb(V)-(III) | Sb2O5+6H++4e-=2SbO++3H2O | 0.581 |
Te(IV)-(0) | TeO2+4H++4e-=Te+2H2O | 0.593 |
U(V)-(IV) | UO2++4H++e-=U4++2H2O | 0.612 |
**Hg(II)-(I) | 2HgCl2+2e-=Hg2Cl2+2Cl- | 0.63 |
Pt(IV)-(II) | [PtCl6]2-+2e-=[PtCl4]2-+2Cl- | 0.68 |
O(0)-(-I) | O2+2H++2e-=H2O2 | 0.695 |
Pt(II)-(0) | [PtCl4]2-+2e-=Pt+4Cl- | 0.755 |
*Se(IV)-(0) | H2SeO3+4H++4e-=Se+3H2O | 0.74 |
Fe(III)-(II) | Fe3++e-=Fe2+ | 0.771 |
Hg(I)-(0) | Hg22++2e-=2Hg | 0.7973 |
Ag(I)-(0) | Ag++e-=Ag | 0.7996 |
Os(VIII)-(0) | OsO4+8H++8e-=Os+4H2O | 0.8 |
N(V)-(IV) | 2NO3-+4H++2e-=N2O4+2H2O | 0.803 |
Hg(II)-(0) | Hg2++2e-=Hg | 0.851 |
Si(IV)-(0) | SiO2(石英)+4H++4e-=Si+2H2O | 0.857 |
Cu(II)-(I) | Cu2++I-+e-=CuI | 0.86 |
N(III)-(I) | 2HNO2+4H++4e-=H2N2O2+2H2O | 0.86 |
Hg(II)-(I) | 2Hg2++2e-=Hg22+ | 0.920 |
N(V)-(III) | NO3-+3H++2e-=HNO2+H2O | 0.934 |
Pd(II)-(0) | Pd2++2e-=Pd | 0.951 |
N(V)-(II) | NO3-+4H++3e-=NO+2H2O | 0.957 |
N(III)-(II) | HNO2+H++e-=NO+H2O | 0.983 |
I(I)-(-I) | HIO+H++2e-=I-+H2O | 0.987 |
V(V)-(IV) | VO2++2H++e-=VO2++H2O | 0.991 |
V(V)-(IV) | V(OH)4++2H++e-=VO2++3H2O | 1.00 |
Au(III)-(0) | [AuCl4]-+3e-=Au+4Cl- | 1.002 |
Te(VI)-(IV) | H6TeO6+2H++2e-=TeO2+4H2O | 1.02 |
N(IV)-(II) | N2O4+4H++4e-=2NO+2H2O | 1.035 |
N(IV)-(III) | N2O4+2H++2e-=2HNO2 | 1.065 |
I(V)-(-I) | IO3-+6H++6e-=I-+3H2O | 1.085 |
Br(0)-(-I) | Br2(aq)+2e-=2Br- | 1.0873 |
Se(VI)-(IV) | SeO42-+4H++2e-=H2SeO3+H2O | 1.151 |
Cl(V)-(IV) | ClO3-+2H++e-=ClO2+H2O | 1.152 |
Pt(II)-(0) | Pt2++2e-=Pt | 1.18 |
Cl(VII)-(V) | ClO4-+2H++2e-=ClO3-+H2O | 1.189 |
I(V)-(0) | 2IO3-+12H++10e-=I2+6H2O | 1.195 |
Cl(V)-(III) | ClO3-+3H++2e-=HClO2+H2O | 1.214 |
Mn(IV)-(II) | MnO2+4H++2e-=Mn2++2H2O | 1.224 |
O(0)-(-II) | O2+4H++4e-=2H2O | 1.229 |
Tl(III)-(I) | Tl3++2e-=Tl+ | 1.252 |
Cl(IV)-(III) | ClO2+H++e-=HClO2 | 1.277 |
N(III)-(I) | 2HNO2+4H++4e-=N2O+3H2O | 1.297 |
**Cr(VI)-(III) | Cr2O72-+14H++6e-=2Cr3++7H2O | 1.33 |
Br(I)-(-I) | HBrO+H++2e-=Br+H2O | 1.331 |
Cr(VI)-(III) | HCrO4-+7H++3e-=Cr3++4H2O | 1.350 |
Cl(0)-(-I) | Cl2(g)+2e-=2Cl- | 1.35827 |
Cl(VII)-(-I) | ClO4-+8H++8e-=Cl-+4H2O | 1.389 |
Cl(VII)-(0) | 2ClO4-+16H++14e-=Cl2+8H2O | 1.39 |
Au(III)-(I) | Au3++2e-=Au+ | 1.401 |
Br(V)-(-I) | BrO3-+6H++6e-=Br-+3H2O | 1.423 |
I(I)-(0) | 2HIO+2H++2e-=I2+2H2O | 1.439 |
Cl(V)-(-I) | ClO3-+6H++6e-=Cl-+3H2O | 1.451 |
Pb(IV)-(II) | PbO2+4H++2e-=Pb2++2H2O | 1.455 |
Cl(V)-(0) | 2ClO3-+12H++10e-=Cl2+6H2O | 1.47 |
Cl(I)-(-I) | HClO+H++2e-=Cl-+H2O | 1.482 |
Br(V)-(0) | 2BrO3-+12H++10e-=Br2+6H2O | 1.482 |
Au(III)-(0) | Au3++3e-=Au | 1.498 |
Mn(VII)-(II) | MnO4-+8H++5e-=Mn2++4H2O | 1.507 |
Mn(III)-(II) | Mn3++e-=Mn2+ | 1.5415 |
Cl(III)-(-I) | HClO2+3H++4e-=Cl-+2H2O | 1.570 |
Br(I)-(0) | 2HBrO+2H++2e-=Br2(aq)+2H2O | 1.574 |
N(II)-(I) | 2NO+2H++2e-=N2O+H2O | 1.591 |
I(VII)-(V) | H5IO6+H++2e-=IO3-+3H2O | 1.601 |
Cl(I)-(0) | 2HClO+2H++2e-=Cl2+2H2O | 1.611 |
Cl(III)-(I) | HClO2+2H++2e-=HClO+H2O | 1.645 |
Ni(IV)-(II) | NiO2+4H++2e-=Ni2++2H2O | 1.678 |
Mn(VII)-(IV) | MnO4-+4H++3e-=MnO2+2H2O | 1.679 |
Pb(IV)-(II) | PbO2+SO42-+4H++2e-=PbSO4+2H2O | 1.6913 |
Au(I)-(0) | Au++e-=Au | 1.692 |
Ce(IV)-(III) | Ce4++e-=Ce3+ | 1.72 |
N(I)-(0) | N2O+2H++2e-=N2+H2O | 1.766 |
O(-I)-(-II) | H2O2+2H++2e-=2H2O | 1.776 |
Co(III)-(II) | Co3++e-=Co2+(2mol·L-1H2SO4) | 1.83 |
Ag(II)-(I) | Ag2++e-=Ag+ | 1.980 |
S(VII)-(VI) | S2O82-+2e-=2SO42- | 2.010 |
O(0)-(-II) | O3+2H++2e-=O2+H2O | 2.076 |
O(II)-(-II) | F2O+2H++4e-=H2O+2F- | 2.153 |
Fe(VI)-(III) | FeO42-+8H++3e-=Fe3++4H2O | 2.20 |
O(0)-(-II) | O(g)+2H++2e-=H2O | 2.421 |
F(0)-(-I) | F2+2e-=2F- | 2.866 |
F(0)-(-I) | F2+2H++2e-=2HF | 3.053 |
在鹼性溶液中(298K)
電對 | 方程式 | Eq/V |
Ca(II)-(0) | Ca(OH)2+2e-=Ca+2OH- | -3.02 |
Ba(II)-(0) | Ba(OH)2+2e-=Ba+2OH- | -2.99 |
La(III)-(0) | La(OH)3+3e-=La+3OH- | -2.90 |
Sr(II)-(0) | Sr(OH)2·8H2O+2e-=Sr+2OH-+8H2O | -2.88 |
Mg(II)-(0) | Mg(OH)2+2e-=Mg+2OH- | -2.690 |
Be(II)-(0) | Be2O32-+3H2O+4e-=2Be+6OH- | -2.63 |
Hf(IV)-(0) | HfO(OH)2+H2O+4e-=Hf+4OH- | -2.50 |
Zr(IV)-(0) | H2ZrO3+H2O+4e-=Zr+4OH- | -2.36 |
Al(III)-(0) | H2AlO3-+H2O+3e-=Al+4OH- | -2.33 |
P(I)-(0) | H2PO2-+e-=P+2OH- | -1.82 |
B(III)-(0) | H2BO3-+H2O+3e-=B+4OH- | -1.79 |
P(III)-(0) | HPO32-+2H2O+3e-=P+5OH- | -1.71 |
Si(IV)-(0) | SiO32-+3H2O+4e-=Si+6OH- | -1.697 |
P(III)-(I) | HPO32-+2H2O+2e-=H2PO2-+3OH- | -1.65 |
Mn(II)-(0) | Mn(OH)2+2e-=Mn+2OH- | -1.56 |
Cr(III)-(0) | Cr(OH)3+3e-=Cr+3OH- | -1.48 |
*Zn(II)-(0) | [Zn(CN)4]2-+2e-=Zn+4CN- | -1.26 |
Zn(II)-(0) | ZnO+H2O+2e-=Zn+2OH- | -1.249 |
Ga(III)-(0) | H2GaO3-+H2O+3e-=Ga+4OH- | -1.219 |
Zn(II)-(0) | ZnO22-+2H2O+2e-=Zn+4OH- | -1.215 |
Cr(III)-(0) | CrO2-+2H2O+3e-=Cr+4OH- | -1.2 |
Te(0)-(-I) | Te+2e-=Te2- | -1.143 |
P(V)-(III) | PO43-+2H2O+2e-=HPO32-+3OH- | -1.05 |
*Zn(II)-(0) | [Zn(NH3)4]2++2e-=Zn+4NH3 | -1.04 |
*W(VI)-(0) | WO42-+4H2O+6e-=W+8OH- | -1.01 |
*Ge(IV)-(0) | HGeO3-+2H2O+4e-=Ge+5OH- | -1.0 |
Sn(IV)-(II) | [Sn(OH)6]2-+2e-=HSnO2-+H2O+3OH- | -0.93 |
S(VI)-(IV) | SO42-+H2O+2e-=SO32-+2OH- | -0.93 |
Se(0)-(-II) | Se+2e-=Se2- | -0.924 |
Sn(II)-(0) | HSnO2-+H2O+2e-=Sn+3OH- | -0.909 |
P(0)-(-III) | P+3H2O+3e-=PH3(g)+3OH- | -0.87 |
N(V)-(IV) | 2NO3-+2H2O+2e-=N2O4+4OH- | -0.85 |
H(I)-(0) | 2H2O+2e-=H2+2OH- | -0.8277 |
Cd(II)-(0) | Cd(OH)2+2e-=Cd(Hg)+2OH- | -0.809 |
Co(II)-(0) | Co(OH)2+2e-=Co+2OH- | -0.73 |
Ni(II)-(0) | Ni(OH)2+2e-=Ni+2OH- | -0.72 |
As(V)-(III) | AsO43-+2H2O+2e-=AsO2-+4OH- | -0.71 |
Ag(I)-(0) | Ag2S+2e-=2Ag+S2- | -0.691 |
As(III)-(0) | AsO2-+2H2O+3e-=As+4OH- | -0.68 |
Sb(III)-(0) | SbO2-+2H2O+3e-=Sb+4OH- | -0.66 |
*Re(VII)-(IV) | ReO4-+2H2O+3e-=ReO2+4OH- | -0.59 |
*Sb(V)-(III) | SbO3-+H2O+2e-=SbO2-+2OH- | -0.59 |
Re(VII)-(0) | ReO4-+4H2O+7e-=Re+8OH- | -0.584 |
*S(IV)-(II) | 2SO32-+3H2O+4e-=S2O32-+6OH- | -0.58 |
Te(IV)-(0) | TeO32-+3H2O+4e-=Te+6OH- | -0.57 |
Fe(III)-(II) | Fe(OH)3+e-=Fe(OH)2+OH- | -0.56 |
S(0)-(-II) | S+2e-=S2- | -0.47627 |
Bi(III)-(0) | Bi2O3+3H2O+6e-=2Bi+6OH- | -0.6 |
N(III)-(II) | NO2-+H2O+e-=NO+2OH- | -0.46 |
*Co(II)-(0) | [Co(NH3)6]2++2e-=Co+6NH3 | -0.422 |
Se(IV)-(0) | SeO32-+3H2O+4e-=Se+6OH- | -0.366 |
Cu(I)-(0) | Cu2O+H2O+2e-=2Cu+2OH- | -0.360 |
Tl(I)-(0) | TlOH+e-=Tl+OH- | -0.34 |
*Ag(I)-(0) | [Ag(CN)2]-+e-=Ag+2CN- | -0.31 |
Cu(II)-(0) | Cu(OH)2+2e-=Cu+2OH- | -0.222 |
Cr(VI)-(III) | CrO42-+4H2O+3e-=Cr(OH)3+5OH- | -0.13 |
*Cu(I)-(0) | [Cu(NH3)2]++e-=Cu+2NH3 | -0.12 |
O(0)-(-I) | O2+H2O+2e-=HO2-+OH- | -0.076 |
Ag(I)-(0) | AgCN+e-=Ag+CN- | -0.017 |
N(V)-(III) | NO3-+H2O+2e-=NO2-+2OH- | 0.01 |
Se(VI)-(IV) | SeO42-+H2O+2e-=SeO32-+2OH- | 0.05 |
Pd(II)-(0) | Pd(OH)2+2e-=Pd+2OH- | 0.07 |
S(II,V)-(II) | S4O62-+2e-=2S2O32- | 0.08 |
Hg(II)-(0) | HgO+H2O+2e-=Hg+2OH- | 0.0977 |
Co(III)-(II) | [Co(NH3)6]3++e-=[Co(NH3)6]2+ | 0.108 |
Pt(II)-(0) | Pt(OH)2+2e-=Pt+2OH- | 0.14 |
Co(III)-(II) | Co(OH)3+e-=Co(OH)2+OH- | 0.17 |
Pb(IV)-(II) | PbO2+H2O+2e-=PbO+2OH- | 0.247 |
I(V)-(-I) | IO3-+3H2O+6e-=I-+6OH- | 0.26 |
Cl(V)-(III) | ClO3-+H2O+2e-=ClO2-+2OH- | 0.33 |
Ag(I)-(0) | Ag2O+H2O+2e-=2Ag+2OH- | 0.342 |
Fe(III)-(II) | [Fe(CN)6]3-+e-=[Fe(CN)6]4- | 0.358 |
Cl(VII)-(V) | ClO4-+H2O+2e-=ClO3-+2OH- | 0.36 |
*Ag(I)-(0) | [Ag(NH3)2]++e-=Ag+2NH3 | 0.373 |
O(0)-(-II) | O2+2H2O+4e-=4OH- | 0.401 |
I(I)-(-I) | IO-+H2O+2e-=I-+2OH- | 0.485 |
*Ni(IV)-(II) | NiO2+2H2O+2e-=Ni(OH)2+2OH- | 0.490 |
Mn(VII)-(VI) | MnO4-+e-=MnO42- | 0.558 |
Mn(VII)-(IV) | MnO4-+2H2O+3e-=MnO2+4OH- | 0.595 |
Mn(VI)-(IV) | MnO42-+2H2O+2e-=MnO2+4OH- | 0.60 |
Ag(II)-(I) | 2AgO+H2O+2e-=Ag2O+2OH- | 0.607 |
Br(V)-(-I) | BrO3-+3H2O+6e-=Br-+6OH- | 0.61 |
Cl(V)-(-I) | ClO3-+3H2O+6e-=Cl-+6OH- | 0.62 |
Cl(III)-(I) | ClO2-+H2O+2e-=ClO-+2OH- | 0.66 |
I(VII)-(V) | H3IO62-+2e-=IO3-+3OH- | 0.7 |
Cl(III)-(-I) | ClO2-+2H2O+4e-=Cl-+4OH- | 0.76 |
Br(I)-(-I) | BrO-+H2O+2e-=Br-+2OH- | 0.761 |
Cl(I)-(-I) | ClO-+H2O+2e-=Cl-+2OH- | 0.841 |
*Cl(IV)-(III) | ClO2(g)+e-=ClO2- | 0.95 |
O(0)-(-II) | O3+H2O+2e-=O2+2OH- | 1.24 |
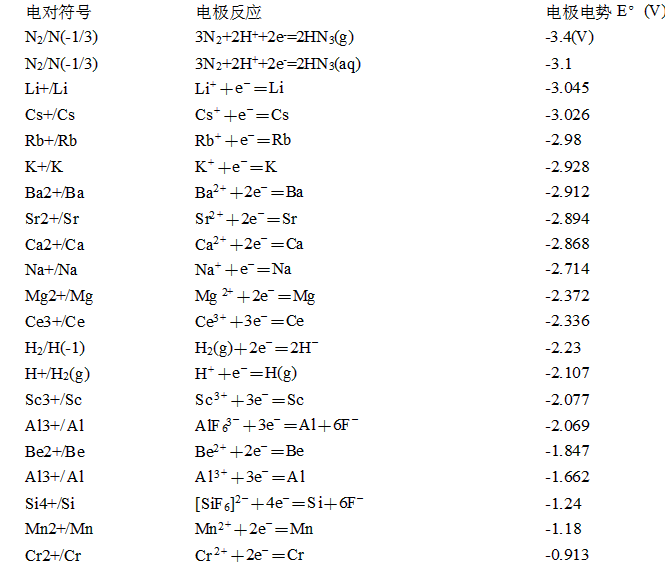
標準電極電勢表
E°(V) | 來源 | |
3N2(g)+2H++2e-=2HN3(aq) | -3.09 | [6] |
Li++e-=Li(s) | -3.0401 | [5] |
N2(g)+4H2O+2e-=2NH2OH(aq)+2OH- | -3.04 | [6] |
K++e-=K(s) | -2.931 | [5] |
Rb++e-=Rb(s) | -2.927 | [4] |
Cs++e-=Cs(s) | -2.925 | [5] |
Ba2++2e-=Ba(s) | -2.912 | [5] |
La(OH)3(s)+3e-=La(s)+3OH- | -2.90 | [5] |
Sr2++2e-=Sr(s) | -2.899 | [5] |
Ca2++2e-=Ca(s) | -2.868 | [5] |
Eu2++2e-=Eu(s) | -2.812 | [5] |
Ra2++2e-=Ra(s) | -2.8 | [5] |
Na++e-=Na(s) | -2.71 | [5][9] |
La3++3e-=La(s) | -2.379 | [5] |
Y3++3e-=Y(s) | -2.372 | [5] |
Mg2++2e-=Mg(s) | -2.372 | [5] |
ZrO(OH)2(s)+H2O+4e-=Zr(s)+4OH- | -2.36 | [5] |
Al(OH)4-+3e-=Al(s)+4OH- | -2.33 | |
Al(OH)3(s)+3e-=Al(s)+3OH- | -2.31 | |
H2(g)+2e-=2H- | -2.25 | |
Ac3++3e-=Ac(s) | -2.20 | |
Be2++2e-=Be(s) | -1.85 | |
U3++3e-=U(s) | -1.66 | [7] |
Al3++3e-=Al(s) | -1.66 | [9] |
Ti2++2e-=Ti(s) | -1.63 | [9] |
ZrO2(s)+4H++4e-=Zr(s)+2H2O | -1.553 | [5] |
Zr4++4e-=Zr(s) | -1.45 | [5] |
TiO(s)+2H++2e-=Ti(s)+H2O | -1.31 | |
Ti2O3(s)+2H++2e-=2TiO(s)+H2O | -1.23 | |
Ti3++3e-=Ti(s) | -1.21 | |
Te(s)+2e-=Te2- | -1.143 | [2] |
V2++2e-=V(s) | -1.13 | |
Nb3++3e-=Nb(s) | -1.099 | |
Sn(s)+4H++4e-=SnH4(g) | -1.07 | |
Mn2++2e-=Mn(s) | -1.029 | [9] |
SiO2(s)+4H++4e-=Si(s)+2H2O | -0.91 | |
B(OH)3(aq)+3H++3e-=B(s)+3H2O | -0.89 | |
TiO2++2H++4e-=Ti(s)+H2O | -0.86 | |
Bi(s)+3H++3e-=BiH3 | -0.8 | |
2H2O+2e-=H2(g)+2OH- | -0.8277 | [5] |
Zn2++2e-=Zn(Hg) | -0.7628 | [5] |
Zn2++2e-=Zn(s) | -0.7618 | [5] |
Ta2O5(s)+10H++10e-=2Ta(s)+5H2O | -0.75 | |
Cr3++3e-=Cr(s) | -0.74 | |
[Au(CN)2]-+e-=Au(s)+2CN- | -0.60 | |
Ta3++3e-=Ta(s) | -0.6 | |
PbO(s)+H2O+2e-=Pb(s)+2OH- | -0.58 | |
2TiO2(s)+2H++2e-=Ti2O3(s)+H2O | -0.56 | |
Ga3++3e-=Ga(s) | -0.53 | |
U4++e-=U3+ | -0.52 | [7] |
H3PO2(aq)+H++e-=P(白磷)*+2H2O | -0.508 | [5] |
H3PO3(aq)+2H++2e-=H3PO2(aq)+H2O | -0.499 | [5] |
H3PO3(aq)+3H++3e-=P(紅磷)*+3H2O | -0.454 | [5] |
Fe2++2e-=Fe(s) | -0.44 | [9] |
2CO2(g)+2H++2e-=HOOCCOOH(aq) | -0.43 | |
Cr3++e-=Cr2+ | -0.42 | |
Cd2++2e-=Cd(s) | -0.40 | [9] |
GeO2(s)+2H++2e-=GeO(s)+H2O | -0.37 | |
Cu2O(s)+H2O+2e-=2Cu(s)+2OH- | -0.360 | [5] |
PbSO4(s)+2e-=Pb(s)+SO42- | -0.3588 | [5] |
PbSO4(s)+2e-=Pb(Hg)+SO42- | -0.3505 | [5] |
Eu3++e-=Eu2+ | -0.35 | [7] |
In3++3e-=In(s) | -0.34 | [2] |
Tl++e-=Tl(s) | -0.34 | [2] |
Ge(s)+4H++4e-=GeH4(g) | -0.29 | |
Co2++2e-=Co(s) | -0.28 | [5] |
H3PO4(aq)+2H++2e-=H3PO3(aq)+H2O | -0.276 | [5] |
V3++e-=V2+ | -0.26 | [9] |
Ni2++2e-=Ni(s) | -0.25 | |
As(s)+3H++3e-=AsH3(g) | -0.23 | [2] |
MoO2(s)+4H++4e-=Mo(s)+2H2O | -0.15 | |
Si(s)+4H++4e-=SiH4(g) | -0.14 | |
Sn2++2e-=Sn(s) | -0.13 | |
O2(g)+H++e-=HO2·(aq) | -0.13 | |
Pb2++2e-=Pb(s) | -0.13 | [9] |
WO2(s)+4H++4e-=W(s)+2H2O | -0.12 | |
P(紅磷)+3H++3e-=PH3(g) | -0.111 | [5] |
CO2(g)+2H++2e-=HCOOH(aq) | -0.11 | |
Se(s)+2H++2e-=H2Se(g) | -0.11 | |
CO2(g)+2H++2e-=CO(g)+H2O | -0.11 | |
SnO(s)+2H++2e-=Sn(s)+H2O | -0.10 | |
SnO2(s)+2H++2e-=SnO(s)+H2O | -0.09 | |
WO3(aq)+6H++6e-=W(s)+3H2O | -0.09 | [2] |
P(白磷)+3H++3e-=PH3(g) | -0.063 | [5] |
HCOOH(aq)+2H++2e-=HCHO(aq)+H2O | -0.03 | |
2H++2e-=H2(g) | ≡0 | |
S4O62-+2e-=2S2O32- | +0.08 | |
Fe3O4(s)+8H++8e-=3Fe(s)+4H2O | +0.085 | [8] |
N2(g)+2H2O+6H++6e-=2NH4OH(aq) | +0.092 | |
HgO(s)+H2O+2e-=Hg(l)+2OH- | +0.0977 | |
Cu(NH3)42++e-=Cu(NH3)2++2NH3 | +0.10 | [2] |
Ru(NH3)63++e-=Ru(NH3)62+ | +0.10 | [7] |
N2H4(aq)+4H2O+2e-=2NH4++4OH- | +0.11 | |
H2MoO4(aq)+6H++6e-=Mo(s)+4H2O | +0.11 | |
Ge4++4e-=Ge(s) | +0.12 | |
C(s)+4H++4e-=CH4(g) | +0.13 | [2] |
HCHO(aq)+2H++2e-=CH3OH(aq) | +0.13 | |
S(s)+2H++2e-=H2S(g) | +0.14 | |
Sn4++2e-=Sn2+ | +0.15 |
Cu2++e-=Cu+ | +0.159 | [2] |
HSO4-+3H++2e-=SO2(aq)+2H2O | +0.16 | |
UO22++e-=UO2+ | +0.163 | [7] |
SO42-+4H++2e-=SO2(aq)+2H2O | +0.17 | |
TiO2++2H++e-=Ti3++H2O | +0.19 | |
Bi3++2e-=Bi+ | +0.2 | |
SbO++2H++3e-=Sb(s)+H2O | +0.20 | |
H3AsO3(aq)+3H++3e-=As(s)+3H2O | +0.24 | |
GeO(s)+2H++2e-=Ge(s)+H2O | +0.26 | |
UO2++4H++e-=U4++2H2O | +0.273 | [7] |
Re3++3e-=Re(s) | +0.300 | |
Bi3++3e-=Bi(s) | +0.32 | |
VO2++2H++e-=V3++H2O | +0.34 | |
Cu2++2e-=Cu(s) | +0.340 | [2] |
[Fe(CN)6]3-+e-=[Fe(CN)6]4- | +0.36 | |
O2(g)+2H2O+4e-=4OH-(aq) | +0.40 | [9] |
H2MoO4+6H++3e-=Mo3++4H2O | +0.43 | |
Bi++e-=Bi(s) | +0.50 | |
CH3OH(aq)+2H++2e-=CH4(g)+H2O | +0.50 | |
SO2(aq)+4H++4e-=S(s)+2H2O | +0.50 | |
Cu++e-=Cu(s) | +0.520 | [2] |
CO(g)+2H++2e-=C(s)+H2O | +0.52 | |
I2(s)+2e-=2I- | +0.54 | [9] |
I3-+2e-=3I- | +0.53 | [9] |
[AuI4]-+3e-=Au(s)+4I- | +0.56 | |
H3AsO4(aq)+2H++2e-=H3AsO3(aq)+H2O | +0.56 | |
[AuI2]-+e-=Au(s)+2I- | +0.58 | |
MnO4-+2H2O+3e-=MnO2(s)+4OH- | +0.59 | |
S2O32-+6H++4e-=2S(s)+3H2O | +0.60 | |
H2MoO4(aq)+2H++2e-=MoO2(s)+2H2O | +0.65 | |
O2(g)+2H++2e-=H2O2(aq) | +0.70 | |
Tl3++3e-=Tl(s) | +0.72 | |
PtCl62-+2e-=PtCl42-+2Cl- | +0.726 | [7] |
H2SeO3(aq)+4H++4e-=Se(s)+3H2O | +0.74 | |
PtCl42-+2e-=Pt(s)+4Cl- | +0.758 | [7] |
Fe3++e-=Fe2+ | +0.77 | |
Ag++e-=Ag(s) | +0.7996 | [5] |
Hg22++2e-=2Hg(l) | +0.80 | |
NO3-(aq)+2H++e-=NO2(g)+H2O | +0.80 | |
[AuBr4]-+3e-=Au(s)+4Br- | +0.85 | |
Hg2++2e-=Hg(l) | +0.85 | |
MnO4-+H++e-=HMnO4- | +0.90 | |
2Hg2++2e-=Hg22+ | +0.91 | [2] |
Pd2++2e-=Pd(s) | +0.915 | [7] |
[AuCl4]-+3e-=Au(s)+4Cl- | +0.93 | |
MnO2(s)+4H++e-=Mn3++2H2O | +0.95 | |
[AuBr2]-+e-=Au(s)+2Br- | +0.96 | |
Br2(l)+2e-=2Br- | +1.07 | |
Br2(aq)+2e-=2Br- | +1.09 | [9] |
IO3-+5H++4e-=HIO(aq)+2H2O | +1.13 | |
[AuCl2]-+e-=Au(s)+2Cl- | +1.15 | |
HSeO4-+3H++2e-=H2SeO3(aq)+H2O | +1.15 | |
Ag2O(s)+2H++2e-=2Ag(s)+H2O | +1.17 | |
ClO3-+2H++e-=ClO2(g)+H2O | +1.18 | |
Pt2++2e-=Pt(s) | +1.188 | [7] |
ClO2(g)+H++e-=HClO2(aq) | +1.19 | |
2IO3-+12H++10e-=I2(s)+6H2O | +1.20 | |
ClO4-+2H++2e-=ClO3-+H2O | +1.20 | |
O2(g)+4H++4e-=2H2O | +1.23 | [9] |
MnO2(s)+4H++2e-=Mn2++2H2O | +1.23 | |
Tl3++2e-=Tl+ | +1.25 | |
Cl2(g)+2e-=2Cl- | +1.36 | [9] |
Cr2O7- +14H++6e-=2Cr3++7H2O | +1.33 | |
CoO2(s)+4H++e-=Co3++2H2O | +1.42 | |
2NH3OH++H++2e-=N2H5++2H2O | +1.42 | [6] |
2HIO(aq)+2H++2e-=I2(s)+2H2O | +1.44 | |
Ce4++e-=Ce3+ | +1.44 | |
BrO3-+5H++4e-=HBrO(aq)+2H2O | +1.45 | |
β-PbO2(s)+4H++2e-=Pb2++2H2O | +1.460 | [2] |
α-PbO2(s)+4H++2e-=Pb2++2H2O | +1.468 | [2] |
2BrO3-+12H++10e-=Br2(l)+6H2O | +1.48 | |
2ClO3-+12H++10e-=Cl2(g)+6H2O | +1.49 | |
MnO4-+8H++5e-=Mn2++4H2O | +1.51 | |
HO2·+H++e-=H2O2(aq) | +1.51 | |
Au3++3e-=Au(s) | +1.52 | |
NiO2(s)+2H++2e-=Ni2++2OH- | +1.59 | |
2HClO(aq)+2H++2e-=Cl2(g)+2H2O | +1.63 | |
Ag2O3(s)+6H++4e-=2Ag++3H2O | +1.67 | |
HClO2(aq)+2H++2e-=HClO(aq)+H2O | +1.67 | |
Pb4++2e-=Pb2+ | +1.69 | [2] |
MnO4-+4H++3e-=MnO2(s)+2H2O | +1.70 | |
H2O2(aq)+2H++2e-=2H2O | +1.78 | |
AgO(s)+2H++e-=Ag++H2O | +1.77 | |
Co3++e-=Co2+ | +1.82 | |
Au++e-=Au(s) | +1.83 | [2] |
BrO4-+2H++2e-=BrO3-+H2O | +1.85 | |
Ag2++e-=Ag+ | +1.98 | [2] |
S2O82-+2e-=2SO42- | +2.07 | |
O3(g)+2H++2e-=O2(g)+H2O | +2.075 | [7] |
HMnO4-+3H++2e-=MnO2(s)+2H2O | +2.09 | |
F2(g)+2e-=2F- | +2.87 | [2][9] |
F2(g)+2H++2e-=2HF(aq) | +3.05 | [2] |