吡啶-3,4-二甲酸酐是一種化學品。
基本介紹
- 中文名:吡啶-3,4-二甲酸酐
- 外文名:Pyridine-3,4-dicarboxylic anhydride
- CAS號:4664-08-8
- 分子式:C7H3NO3
基本信息,目標產物,合成方法,英文介紹,參考資料,
基本信息
中文名稱: | 吡啶-3,4-二甲酸酐 |
中文別名: | 吡啶-3,4-二羧酸酐 |
英文名稱: | - |
英文別名: | -; furo[3,4-c]pyridine-1,3-dione |
分子量: | 149.1036 |
InChI: | InChI=1/C7H3NO3/c9-6-4-1-2-8-3-5(4)7(10)11-6/h1-3H |
密度: | 1.556g/cm3 |
沸點: | 334.6°C at 760 mmHg |
閃點: | 156.2°C |
蒸汽壓: | 0.000127mmHg at 25°C |
目標產物
Pixantrone maleate, BBR-2778
合成方法
英文介紹
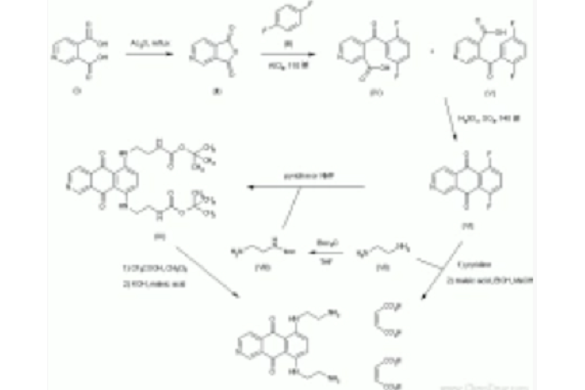
3,4-Pyridinedicarboxylic acid (I) was converted to the cyclic anhydride (II) upon heating with acetic anhydride. Friedel-Crafts condensation of anhydride (II) with p-difluorobenzene (III) in the presence of AlCl3 gave rise to a mixture of two regioisomeric keto acids, (IV) and (V). Cyclization of this mixture in fuming sulfuric acid at 140 C generated the benzoisoquinoline (VI) (1,2). Subsequent displacement of the fluorine atoms of (VI) with ethylenediamine (VII) in pyridine provided the target bis(2-aminoethylamino) derivative, which was finally converted to the stable dimaleate salt. Alternatively, ethylenediamine (VII) was protected as the mono-N-Boc derivative (VIII) by treatment with Boc2O. Condensation of the difluoro compound (VI) with the protected ethylenediamine (VIII) furnished (IX). The Boc groups of (IX) were then removed by treatment with trifluoroacetic acid. After adjustment of the pH to 4.2 with KOH, treatment with maleic acid provided BBR-2778.
參考資料
Krapcho, A.P.; et al.; 6,9-Bis[(aminoalkyl)amino]benzo[g]isoquinoline-5, 10-diones. A novel class of chromophore-modified antitumor anthracene-9,10-diones: Synthesis and antitumor evaluations. J Med Chem 1994, 37, 6, 828